[Feature] Breaking Fibrin Barriers: A New Approach to Boost Immune Response in Pancreatic Cancer
Acollaborative research team led by Choi Jeong-uk, a professor in the College of Pharmacy at Kyung Hee University, and Taslim A. Al-Hilal, a professor in the Department of Molecular Pharmaceutics and Biomedical Engineering at the University of Utah, uncovered how fibrin in pancreatic ductal adenocarcinoma (PDAC) affects immune cell behavior. Their study found that fibrin forms a barrier that hinders immune cell infiltration, enabling the cancer to evade immune attacks. The research was published in Biomaterials on March 20, 2025, under the title, Stromal fibrin shapes immune infiltration landscape of pancreatic ductal adenocarcinoma.
Key Points of Research: FibTS
Pancreatic cancer has one of the lowest five-year survival rates, hovering around 15%, largely due to its lack of early symptoms and the difficulty in early detection. The most prevalent form, PDAC, accounts for 85–90% of all cases. The research identified that within PDAC, blood clotting leads to the accumulation of fibrin—a fibrous protein—which forms a dense structure known as crosslinked fibrin-rich tumor stroma (FibTS). This structure influences the movement of immune cells within the tumor.
The research team investigated how FibTS shapes the behavior of immune cells within pancreatic tumors. They discovered that FibTS acts as a physical and immunological barrier, blocking the entry of key immune cells—particularly CD8+ T cells and tumor-associated macrophages. This restricted access fosters an immunosuppressive environment, allowing cancer cells to evade immune surveillance and continue growing. Importantly, the researchers demonstrated that pharmacologically disrupting FibTS formation reshaped immune cell infiltration patterns and slowed PDAC progression, highlighting a potential new therapeutic strategy.
Prof. Choi has long studied blood clotting and initially focused on its mechanisms during his doctoral research. His interest turned to cancer upon observing that blood clots frequently form around solid tumors, as cancer cells damage blood vessels and promote metastasis. This led him to explore clot removal as a cancer treatment, which both suppressed tumor growth and improved the immune environment—laying the foundation for the FibTS concept.
In collaboration with Prof. Al-Hilal’s research team, which specializes in microfluidics, the researchers developed a tumor-mimicking microfluidic device that simulated the flow of blood-like fluid. Using this system, they found that cancer-fighting immune cells—such as CD8+ T cells and M1 macrophages—were impeded in their movement, while M2 macrophages, known to promote tumor growth, were able to infiltrate and take shelter within the clot-like matrix. “We do not yet know why,” Prof. Choi said, “but it is fascinating that clot formation appears to have a selective mechanism—favoring immune cells that support tumor growth, while excluding those that suppress it.”
Fibrin in blood clotting
Photo: Britannica (britannica.com)
Background of Research
Pancreatic cancer is notorious for its low survival rate and resistance to conventional treatments, including surgery. One major obstacle is its uniquely dense and pressurized tumor microenvironment, primarily composed of collagen-rich stroma. This fibrotic barrier limits immune cell penetration and severely undermines the efficacy of immunotherapy and other anticancer drugs.
Prof. Choi, whose background is in engineering, began this research with the aim of applying engineering principles to cancer treatment. Since previous studies have shown that blood clots occur more frequently in the microenvironment of pancreatic cancer than in other cancer types, the team focused on pancreatic cancer as the ideal model. Their goal was to know how targeting clot formation could enhance the effectiveness of cancer therapies.
Significance and Prospect of Research: Turning Clots into a Cure
The research revealed that FibTS in pancreatic cancer acts as a barrier, blocking immune cells from entering the tumor. Crucially, selectively inhibiting this fibrin structure slowed tumor growth. By identifying the dense, crosslinked structure of fibrin proteins formed during blood clotting as a key component of the tumor microenvironment, the researchers proposed a new combination therapy strategy: pairing anticancer drugs with antithrombotic agents. This approach could enhance the infiltration of both immune cells and therapeutic drugs, offering a more effective path for treatment.
Prof. Choi emphasized that the significance of this study lies in its innovative yet practical approach—rather than departing drastically from existing cancer treatments, it introduces antithrombotic drugs as a new therapeutic angle. The concept of using antithrombotic agents, commonly referred to as blood thinners, to treat cancer is relatively novel, offering a promising alternative that enhances clinical feasibility by allowing combination with various anticancer therapies. This strategy broadens therapeutic options while also creating potential for synergistic effects. Importantly, its application may extend beyond pancreatic cancer to other major solid tumors such as gastric, lung, and liver cancers. Antithrombotic therapy could also serve as a valuable second or third-line treatment option when standard therapies fail.
However, Prof. Choi emphasized that further research is needed before FibTS-targeted therapies can be applied in clinical settings. Research on dosing and treatment intervals is essential, as preventive use of antithrombotic drugs could pose long-term risks such as bleeding. He also pointed out tumor heterogeneity—the fact that cancer can vary not only between patients but also within a single tumor. This makes FibTS-targeted therapy unsuitable in some cases, and this approach may be difficult to apply where clots are sparse or in non-solid cancers like blood cancers. Nevertheless, he remains optimistic about its potential as an effective treatment for solid tumors where FibTS is clearly present.
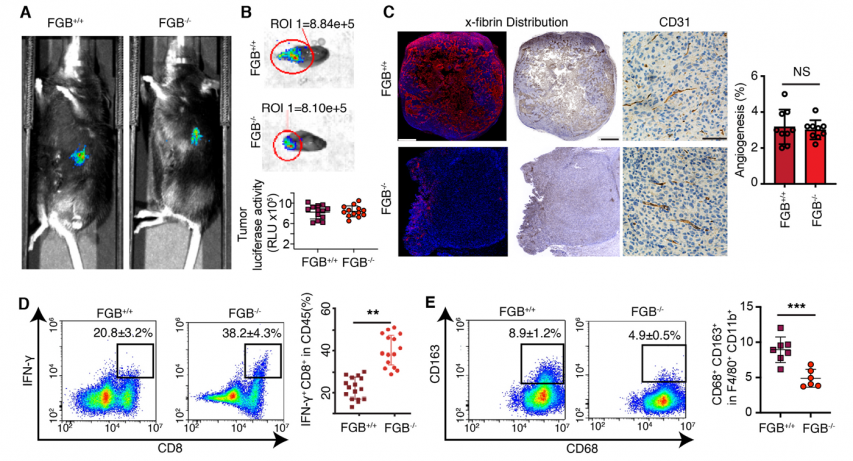
Knockdown fibrinogen genes reverses the immunosuppressive microenvironment of tumors
Photo: ScienceDirect (sciencedirect.com)
FibTS: A New Way to Unlocking Immunotherapy
The research reveals that FibTS in PDAC acts as a barrier to immune cell infiltration and it enables the tumor to evade immune surveillance. It also demonstrates that selectively inhibiting FibTS can restore immune activity and slow tumor growth. This discovery is especially significant as it introduces FibTS as a novel immunotherapeutic target—not only for pancreatic cancer but potentially for a wide range of solid tumors. It offers a new avenue to enhance the effectiveness of cancer immunotherapy.
There are no registered comments.
I agree to the collection of personal information. [view]